Osmosis Study Guide Answers

Diffusion and Osmosis Have you ever ridden in a crowded elevator? When the doors open, everyone hurries out of the car, eager to get away from each other as quickly as possible. When molecules are confined into a small area and are given a way to get away from each other, they do so. Not because they're hot and annoyed but because of diffusion. Diffusion is the movement of molecules from an area of high concentration to low concentration due to molecular kinetic energy; that is, the endless and random movement of molecules.
Osmosis is a specialized type of diffusion: the diffusion of water. In both diffusion and osmosis, materials move down a concentration gradient, the difference in the number of molecules between two areas. You can think of a concentration gradient as a hill, with the top of the hill being an area of high concentration, the bottom of the hill as an area of low concentration, and the angle of the hill being the concentration gradient. The steeper the hill, the faster objects roll down it; the steeper the concentration gradient, the faster molecules move from high to low concentration. Diffusion and osmosis are directly affected by the ratio of a cell's volume to its surface area. An increase in this ratio means an increase in the rate of diffusion.
Let's use cubes to represent cells. A smaller cube will have higher surface area to volume ratio than a larger cube. This means that more of the cell's interior is exposed to molecules outside of the cell. The opposite is true in larger cells - less of the cell's interior is close to the cell's environment. These differences mean both more and faster diffusion occurs in smaller cells than in larger cells. Differential Permeability Diffusion and osmosis are important in helping cells to create homeostasis, a stable internal environment, inside of the cell membrane.
There are several types of diffusion. Simple diffusion is defined as movement of molecules across a membrane by a concentration gradient, while facilitated diffusion occurs if molecules cross the membrane via a protein channel or carrier. Active transport happens when molecules are pushed against the concentration gradient. This requires energy. These different types of diffusion make the cell's membranes selectively permeable, which means the cell membrane helps control what materials enter and exit the cell. We can demonstrate selective permeability by creating model cells using dialysis tubing, a material used in hospitals to mimic kidney function in patients that have kidney disease. Dialysis tubing is selectively permeable to common organic molecules based on size.
Molecules (like glucose and water) and ions (like sodium) pass through readily, while large molecules (like sucrose) do not. Let's see what happens when we create artificial cells made of dialysis tubing. We'll create four conditions. Condition one will be our control condition, since it's water both inside and outside of the cell. Condition two will be our first experimental condition, with a one molar sucrose solution inside the cell and distilled water outside the cell.
Condition three will be another experimental condition, with a five molar sucrose solution inside the cell and distilled water outside of the cell. Condition four will use the same solutions as in condition three. This time, though, we'll put the distilled water inside of the cell and the sucrose solution outside of the cell. This condition will provide evidence of what happens when the solutions are reversed and assure us that any movement of solutions due to osmosis can happen in either direction. Condition Number Cell contents Beaker contents 1 distilled water distilled water 2 1M sucrose distilled water 3 5M sucrose distilled water 4 distilled water 5M sucrose If we observe the cells at the beginning of the experiment and weighed them at the end of an hour, what would you expect to see?
New Holland Ford 4600 4610 4630 Tractor Service Repair Manual| eBooks| Technical. Double clutches, non-synchromesh transmission, gearbox, shaft, rear. Complete Service Repair Manual For Ford New Holland 10 & 30 series. Eight Speed Synchromesh Transmissions Ford 2610, 3610, 4110 & 4610. Repair manual for a ford 4610 transmission. New Ford Shop Manual for Tractor Models 2610 3600 3610 4110 4600 4610. EIGHT SPEED NON-SYNCHROMESH TRANSMISSIONS-FORD 2610, 3610,. This is the complete 1000+ repair manual for Ford tractor models 2600, 3600. 3 - Eight Speed Synchromesh Transmissions - Ford 2610, 3610, 4110 & 4610. Buy now repair manual New Holland Ford 4610 Tractor Repair Manual PDF. 3 - Eight Speed Synchromesh Transmissions - Ford 2610, 3610, 4110 and 4610.
Water will move from an area of high concentration to low concentration, but sucrose will not move because the artificial cell's membrane is selectively permeable. Furthermore, the greater the concentration gradient between the beaker and the cell, the greater the rate of osmosis. This is called water potential.
Tonicity and Water Potential A cell's cytoplasm is mostly made of water, so we refer to a cell's contents as aqueous. Solid particles, like organic molecules and salts inside of the cell, are solutes. When water moves during osmosis in a cell, we use a special set of terms to clarify which way it's moving. These terms usually refer to how likely it is that water will move from one area to another, called water potential, represented by the Greek letter Psi. As stated at the beginning of the lesson, a high concentration of water molecules in one area means a high water potential. A high concentration of water molecules on one side of a membrane means that there is less room on that side of the membrane for solute.
3.4 Diffusion And Osmosis Study Guide Answers
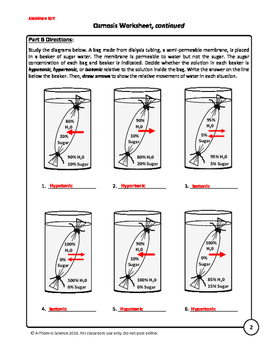
The side of the membrane with a low amount of solute is called hypotonic. Recall that 'hypo' means 'below' or 'beneath,' like a hypodermic needle goes beneath the skin. If there are less water molecules and more solute molecules on one side of a membrane, then the condition is called hypertonic. Recall that 'hyper' means 'high' or 'above,' like how a person might get hyper if they have too much caffeine or sugar. If the concentration of water and solutes are equal on both sides of the membrane, we say it's an isotonic condition. 'Iso' means 'equal,' as in an isosceles triangle, with three equal sides. Water always moves from an area of higher potential to lower potential, so it moves from a hypotonic condition where there's less solute to a hypertonic condition where there's more solute.
Placing an animal cell in liquids that have concentrations of solute different enough to overwhelm a cell's ability to maintain homeostasis can cause the cell to shrink in a hypertonic solution or burst in a hypotonic solution. Plant and some fungal cells have cell walls that help to prevent their cells from losing shape in a hypertonic solution and from bursting in a hypotonic solution. Turgor pressure is defined as the pressure of the water inside the cell against a cell wall. Lesson Summary Let's review!
Diffusion is the movement of molecules from an area of high concentration to low concentration. Osmosis is a specialized type of diffusion: the diffusion of water.

In both diffusion and osmosis, materials move down a concentration gradient, the difference in the number of molecules between two areas - the steeper the concentration gradient, the faster the rate of diffusion. The likelihood that water will move from one area to another down a concentration gradient is called water potential.
A high concentration of water molecules in one area means a high water potential. Different types of diffusion make the cell membranes selectively permeable, which means the cell membrane helps control what materials enter and exit the cell. Selective permeability helps the cell maintain homeostasis, a stable internal environment. Diffusion and osmosis are affected by the ratio of a cell's volume to its surface area. An increase in this ratio means an increase in the rate of diffusion.
This is because more of the cell's interior is exposed to the environment. A cell's environment can be labeled in terms of how much solute and water are present.
A low amount of solute is called hypotonic, while less water molecules and more solute molecules create a hypertonic condition. If the concentration of water and solutes are equal on both sides of the membrane, the cell is in an isotonic condition.
Placing an animal cell in liquids that have concentrations of solute different enough to overwhelm the cell's ability to maintain homeostasis can cause the cell to shrink in a hypertonic solution or burst in a hypotonic solution. Plant and some fungal cells have cell walls that help to prevent their cells from losing shape in a hypertonic solution and from bursting in a hypotonic solution. Turgor pressure is defined as the pressure of the water inside the cell against a cell wall. Learning Outcomes Assess your preparedness to do the following when the lesson ends:.
Write the definitions of diffusion, osmosis, concentration gradient and water potential. Relate the function of selective permeability in the cell membrane.
Realize the impact of the ratio of cell volume to surface area on diffusion and osmosis. Characterize hypotonic, hypertonic and isotonic and understand what can happen when cells are placed in liquid with different solute concentrations.
This page is updated frequently at any time and contains information about books, past and current users, and software. This page also contains links to all known errors in the book, accompanying slides, and software. Because the manual solution is distributed electronically, all known errors are promptly corrected and no error lists are maintained. Instructors are encouraged to visit this site regularly; they may also register on this site to be notified of important changes by email. (read: privacy policy).
Library Archives of.